WHAT IS UNIT DOSE SYSTEM. Those medications which are ordered packaged handled administered and charged in multiples of single dose units containing a predetermined amount of drugs or supply sufficient for one regular dose application or use.

Pin On 2021 Pharmacy Platinum Pages
The use of unit-of-use packaging has increased.

. Studies have shown that patient compliance is most at risk if packaging is hard to open or use. 1 Removing the drugs from the sealed boxes. Unit dose system UDS 3.
Traditional system unit-of-use system and unit dose system. Reduce unnecessary patients costs. Other tasks in the unit dose process are.
A method of preparing medications in which individual doses of patient medications are prepared by the pharmacy and delivered in individual labeled packets. Mostly we talk about the dose to people or to parts of the body but we can define the dose in air water human tissue or any other material. The damage caused by different types of radiation.
Many drug manufacturers and pharmacies in the United States decide not to use unit-of-use packaging mainly because of costs but overall unit-of-use packaging can be more safe and efficient result in less counterfeiting reduce unnecessary costs and ultimately prevent adverse effects. In many cases old age or medical conditions prevent patients from handling pharmaceutical packaging the way the designer or. Easily identify potentially damaged containers.
Authorization bar code quantity of drugs validity etc. When the doctor initiate a prescription for patient this prescription will be brought by a nurse for collection at the inpatient pharmacy or satellite. Single units of dressing materials enable caretakers to use the proper amount of material for each patients needs avoiding waste.
Improved safety for staff and patients. A single unit package is one that contains one discrete pharmaceutical dosage form ie one tablet one 2-mL vol-ume of liquid one 2-g mass of ointment etc. Unit-of-use means a package that provides.
For the pharmaceutical industry unit dose packaging offers limitless possibilities to improve patient compliance. The UDS involves dispensing of drugs to individual patients on a daily basis and for 24 hour duration only. Unit-of-use means a sufficient quantity of a drug for one normal course of therapy.
Energy is most often given in units of ergs erg joules J. This can be done manually or with machines. A unit dose package is one that contains the particular dose of the drug.
1 sievert Sv 100 rem. Unit dosing involves two main processes. A globally accepted dose standard unit is important in drug utilisation studies particularly if different investigations are to be compared.
Unit of use system UoU The unit dose system is defined as a pharmacy coordinated method of dispensing and controlling medications in health care institutions. 1 rem 001 sievert Sv Common Metric Prefixes. Production of single unit and unit dose packages the use of which has been shown to have substantial benefits.
Unit dose packaging eliminates this issue and helps patients save money. Packaging and printing of the label or unit dose package. A unit-of-use package that is a blister package may not be reprocessed by a pharmacist once it has been deblistered from a unit-dose container see General Notices and Requirements for application of the appropriate beyond-use date for a multiple-unit or unit-dose container.
Unit of use system UoU This system involved pharmacist to review individual patients prescription by the prescriber prior to supplying medication to the ward. This system is characterized by medications contained in unit dose packages dispensed in ready-to-administer form and not more than 24-hour supply. Unit-dose medications have been defined as.
1 millirem mrem 0001 rem. 1 microsievert µSv 0000 001 Sv. The unit-of-use system UoU is similar to UDS in many ways except to the duration of supply.
Dosage forms also called unit doses are pharmaceutical drug products in the form in which they are marketed for use with a specific mixture of active ingredients and inactive components in a particular configuration such as a capsule shell for example and apportioned into a particular doseFor example two products may both be amoxicillin but one is in 500 mg capsules and. Deblistering is the process of removing medication from a blister. Electronic documentation for easy monitoring.
View a PDF version of the. Dose Equivalent ¾Measures the Relative Biological Effectiveness RBE of different types of radiation - ie. In radiation protection dose has a more specific meaningit is the energy of ionizing radiation absorbed per unit mass of any material.
¾Converts to a common unit DOSE EQUIVALENT RADIATION ABSORBED DOSE x Q Q Quality factor X-rays gamma rays Q 1 Neutrons Q 10 Alpha particles Q 20 Effective Dose Equivalent or. 1 microrem µrem 0000 001 rem. Hence there is reason to advocate use of the DDD as the sole standard dose unit in all pharmacoepidemiologic studies.
Unit-of-use means a system in which drugs are delivered to the resident areas either in single unit packaging bingo or punch cards blister or strip packs or other system where each drug is physically separate. 2 Drawing up a unit dose order with the corresponding information. Use the Radiation Dose Calculator to estimate your yearly dose from sources of ionizing radiation.
Unit dose system UDS 3. Unit dose packaging has made its way from the medical field to various other industries including cosmetics. 1 millisievert mSv 0001 Sv.
Reduced inaccurate dosing concerns. None of the alternatives seemed to offer any advantage over the DDD. The unit dose system UDS should be encouraged due to its many advantages.
The primary reasons to consider unit dose packaging are. Products such as lotions gels makeup creams and soaps are effectively marketed using this.

Medi Dose Unit Dose Done Right As Seen In The 2017 Platinum Pages Buyer S Guide Rxplatinumpages Com Pharmacy Patient Care Hospital Pharmacy

Ipack Rx Unit Dose Packager By Pearson Medical Technologies Llc Ipack Rx By Pearson Medical Provides A S Hospital Pharmacy Medical Technology Patient Safety

Prepackaged Unit Dose Products By American Health Packaging Bar Coded To The Dose Level Our Unit Dose Line Cont Patient Care Hospital Pharmacy Care Hospital

Pin On Maths For Nurses Drug Unit Conversions

Systane Ultra Unit Dose Vials 60 Ea Systane Ultra Vials Lubricant Eye Drops

Extreme Weather Natural Disaster Morning Message Bundle Digital Print Unit Morning Messages Natural Disasters Extreme Weather

Gain Ultra Flings Original Scent With Febreze Oxi Boost Odor Remover Laundry Detergent 21 Count

Connecting The Pharmacy Profession Medical Packaging Pharmacy Medical

Connecting The Pharmacy Profession Pharmacy Hospital Pharmacy Prescription Vials

Pharmacy Products And Services Pharmacy Hospital Pharmacy Patient Safety

Leader Anti Diarrheal Tablets 12 Count Leader Tablet Anti

Medi Dose Unit Dose Packaging The Way It Should Be As Seen In The 2012 Pharmacy Platinum Pages Buyer S Guide Prescription Vials Dose Nursing Supplies

Medical Packaging Inc Fluidose Series 6 Unit Dose Packaging System
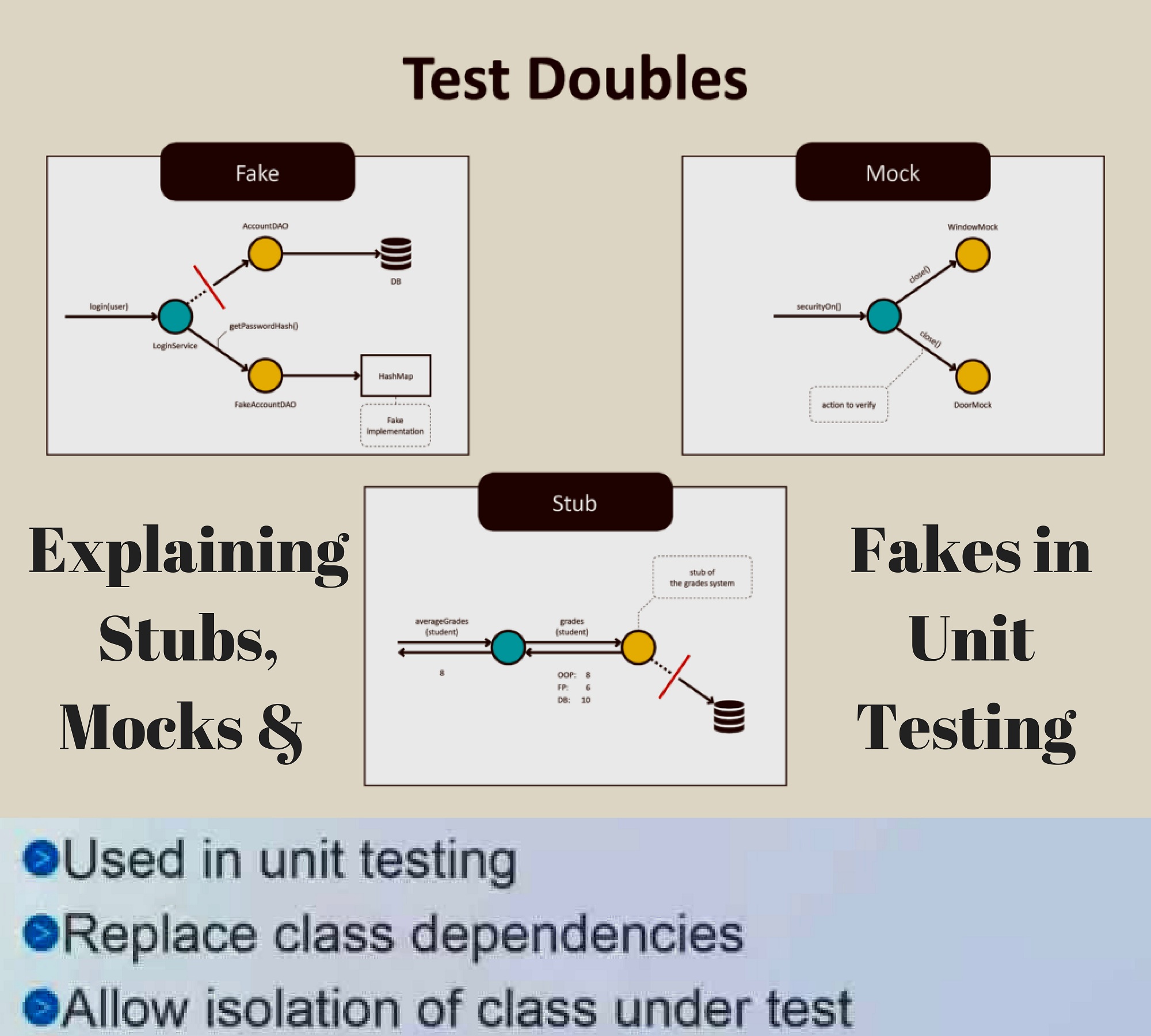
Explaining Stubs Mocks Fakes In Unit Testing Mocking Integration Testing The Unit

Systane Hydration Multi Dose Preservative Free Drops 10ml




